Global Experts in Bio-Containment and Cleanroom Consultancy

Targeted Diagnostics
Producing Engineered Solutions for non-compliant or under-performing Bio-Containment and GMP facilities.
Using our Engineering background coupled with out Regulatory understanding we can evaluate your facility using Targeted Diagnostics that will identify the root cause of your compliance or performance issues then provide solutions to save you time and money.
Non-compliance or performance issues in Bio-Containment or GMP Cleanroom environments can cause significant problems to the facility's operation.
Our Support and Services
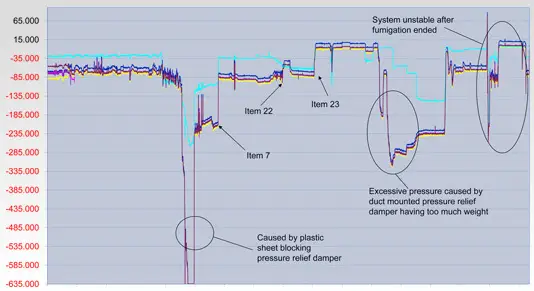
Troubleshooting - Through a structured process of analysis and assessment we are able to interpret the symptoms of your non-conformance or non-compliance and diagnose a clear root cause. Some of the techniques we use are;
Facility / Process Survey and Evaluation - Measuring, collating and evaluating data from throughout the facility, its operational components or the current processes and procedures in order to gain a full understanding of what is happening.
Room Leakage Rates - Repeatable and Quantifiable methodology to analyse and challenge facility construction and components.
Benchmarking & Gap Analysis - Measurement and analysis to provide feedback and a structured route-map back to compliance.
Risk Based Analysis - Utilising the SWIFT (Structured 'What If' Table) analysis to identify potential failure modes, suggest and implement design solutions.
Technical and Regulatory Support - Our combined experience of over 50 years working in Bio-Containment and GMP Cleanroom facility design, construction & operation.
Added Value Solutions - Providing full technical solutions, cost breakdown analysis and potential for improved value and efficiencies.

I have a regulatory compliance issue, how do I overcome this? My facility is under performing / not performing correctly, can this be resolved?
aQmen's targeted diagnostics troubleshoots to the root cause of your performance or compliance issue.